bstract
Within the lifetime of most reverse osmosis (RO) systems some fouling will adversely affect
membrane performance.One of the major foulants identified on lead membranes during a
decade of membrane autopsy at the Genesys Membrane laboratory is biological fouling
(biofouling).
All raw water sources contain microorganisms such as algae, bacteria and fungi. They also
contain compounds which provide nutrients and energy sources which promote biological
growth. In addition current methods of control such as chlorination will increase the
availability of nutrient compounds.
The effects of biofouling on membrane operation include a reduction in flux, increase in
pressure drop and salt passage and potentially membrane degradation and failure. Current
technology favours the use of biocides to control biofouling; however bacteria in biofilm are
more resistant to biocides than planktonic organisms. In addition using biocides produces an
accumulated biomass which encourages active re-growth of the population. Therefore the
successful approach must kill the biological population and successfully remove it from the
membrane surface to prevent rapid re-growth.
This paper explores the processes for developing and testing a cleaning product Genesol 703
which removes biofouling from RO/NF/UF systems. The results of removing biofilm from
membranes are presented. Product efficacy was determined by comparison of membrane flux
rates before and after cleaning and visual inspection by scanning electron microscopy (SEM).
The results demonstrate that Genesol 703 is a technically and economically viable cleaning
chemical product for the removal of biofouling from membranes
1. Introduction
Biofouling is referred to as the undesired development of microbial layers on surfaces [1]. All
raw water sources and therefore reverse osmosis (RO) feed waters contain microorganisms
such as algae, bacteria and fungi, in addition they contain both nutrient and energy sources
which promote growth of the bacterial population.
Biofouling has been recognized as the most serious problem in RO systems [2,3]. Membrane
Autopsy procedures at the Genesys laboratories in Madrid have proved that over a 5 year
period biofouling accounts for 35% of failures of all membranes tested; the most frequently
detected foulant.

Figure 1.The main types of foulant identified on membrane elements from the first position during autopsy
(2001-2007).Source: GMP laboratories statistics
The effects of biofouling on both Brackish water (BWRO) and Sea Water (SWRO) RO
membranes include an increase in pressure drop, decrease in flux, and can also affect salt
passage. In extreme cases membrane degradation and failure can occur. As all of these
consequences will impact directly on operating expenditure a variety of different operating
procedures are regularly implemented during pre-treatment to control the population of
microorganisms in RO feed waters.
Pre-treatment and chemical procedures are required to prevent and control membrane fouling
but have been found to be rarely totally effective in removing microorganisms and nutrients
from the feed water system. In addition differences in RO system design, operating
procedures and the ability of microorganisms to adapt and multiply successfully in membrane
environments makes it difficult to rely on a single method of control. In addition some of the
methods currently in wide use to control biofouling can encourage growth if applied
incorrectly. The following sections give an overview of some of the processes involved in
biofouling and the development of a specific cleaning product. Results from laboratory scale
tests are presented with an explanation of the unique mechanisms which make this product
effective.
2, Background –Membrane biofouling.
All RO feed water sources contain a population of microorganisms and compounds which act
as nutrients or energy sources. It is not the purpose of this paper to outline in detail the
35%
29%
29%
7%
Biofilm&Organic matter
Colloidal/particulate matter
Scales&Inorganic deposits
Not detected
Types of foulants detected in RO membranes
autopsies (2001-2007)
Biofilm&Organic matter Colloidal/particulate matter
Scales&Inorganic deposits Not detected
different types and species of microorganisms which may be present and also the complex
methods of interaction and protection they employ to survive and multiply in the aquatic
membrane environment. A brief description of the microbial populations in the membrane
environment will help explain how current control methods are limited and how a dual
approach is required to control operational problems associated with membrane biofouling.
In RO systems the pre-treatment, pipe work and membrane elements provide a large surface
area for the attachment and growth of free living bacteria entering in the feed water. A
biofilm is described as bacterial aggregates attached to a surface; the biofilm structure
includes a matrix of bacterially produced Extracellular Polymeric Substances (EPS). The EPS
is composed of polysaccharides, proteins and nucleic acids [4] and has been proven to play a
major role in biofouling formation and its behaviour; effectively altering the porosity,
density, water content, charge and sorption properties [1,5] of the biofilm. EPS enhances the
structural integrity and adhesiveness of biofilms through 3 different forces:1. electrostatic, 2.
hydrogen bonds and 3. London dispersion forces [6]. This adhesiveness and elasticity makes
the biofilm difficult to remove from membrane surfaces and also provides protection from
biocides. In addition the presence of divalent cations such as calcium and magnesium
increase the strength of biofilm by forming salt bridges between the membrane surface and
negatively charged bacteria [6].

Figure 2.- Detail of a biofilm fouled membrane with deposit attached to spacer material.
2.1 Methods of Control biofouling
Genesys Membrane Autopsy results prove that biofilm formation does not occur equally
within all areas of the membrane system, as the RO membranes filter out bacteria and
nutrient sources from the feed water the bulk of the biofouling occurs in the first element of a
pressure vessel. However in extreme cases formation can occur on the product side
contaminating permeate water. Time of formation differs widely between RO systems; from
a few days to a few weeks, however in an RO system operating on biologically active feed
water a biofilm will appear within 3-5 days of inoculation [7].
In order to limit biofouling in a membrane system bacteria and nutrient/energy sources are
intended to be removed from the feed water (preventive action). Pre-treatments in RO
facilities have largely evolved in the last years including in some cases membrane
technologies as Microfiltration (MF) or Ultrafiltration (UF). Even these last generation and
extensive pre-treatment designs are rarely a 100% efficient processes and survival of a very
small number of viable cells will lead to multiplication and possible biofouling in the
membranes. In addition to this approach disinfection stages in the pre-treatment system using
biocides or UV are also used. Factors which must be considered when designing a method of
disinfection include feed water quality (particularly; bacteria levels, pH and analysis of both
organic and inorganic compounds), contact time and membrane element type (material). Cost
of biocide application must also be considered as dosage rates will be affected by the size of
the system and level of biofouling.
The chemistry and use of chlorine as a disinfectant is widely covered in literature. It is
extensively used in industrial and municipal applications due to its’ relatively low cost and
widespread availability. It has significant limitations in terms of application in RO systems;
thinfilm composite polyamide membranes are sensitive to levels of chlorine with oxidative
degradation occurring at between 200-1000 hours of exposure to 1ppm of free chlorine [7],
therefore chlorine must be removed from the feed system prior to entering the membrane,
either by activated carbon or dosing Sodium Metabisulphite. Therefore any viable bacterial
population or biofilm in the membrane will not be affected. In addition chlorine breaks down
natural organic matter (NOM) present in the feed water to more easily biodegradable
products offering a nutrient source to micro-organisms. As no chlorine is present on the
membrane surface biofilm growth can occur leading to a requirement for more frequent
sanitization. At this point, it is important to consider the role of EPS in membrane fouling and
performance rather than counts on viable cells on membranes. Within a membrane system a
biofilm level of 10³cfu/cm² for aerobic bacteria is considered normal with operational
problems generally occurring with bacterial counts >105cfu/cm² [8]. Recent studies show that
chlorine-inactivated bacteria may also produce a biofouling layer on the RO membrane
surface [9] .Chlorine is effective in inactivating microorganisms but not in decreasing EPS
concentration, so no improvements in flux decline related to these fouling processes are
observed [5].
Alternative non-oxidising biocides such as DBNPA (2,2-dibromo-3-nitrilopropionamide) and
isothiazalones are approved by membrane manufacturers; however dose rates and therefore
application costs are significantly higher than chlorine making continual dosing impractical.
These chemicals are also not approved for dosing online in potable applications.
Importantly these biocide products are unable to adequately penetrate the protective biofilm
layer and lyse/dissolve these foulants thus preventing re-growth on the membrane [9].
2.2 Membrane Cleaning
Membrane cleaning-in-place (CIP) is also used as a means of biofouling control, primarily
aimed at disrupting and removing the biofilm layer from the membrane system. As reported
by C. Whittaker et al [9] “strongly bactericidal compounds were not necessarily effective in
removing biofouling layers, and cleaning solutions that were effective in biofilm removal
were not necessarily bactericidal”. In any case, disinfections have to be completed by the
removal of the killed cells, which otherwise would adhere to membrane surface.
In practice CIP processes do not fully remove biomass from membranes, particularly in
severe cases when plugging of the feed path restricts transport of cleaning chemicals into the
blocked region. The remaining biomass, rich in nutrients, allows for rapid re-growth after
cleaning. The use of CIP as a means of removing biofilm is often ineffective due to a
combination of incorrect chemical selection – inability to fully penetrate the biofilm layer,
poor cleaning practice (with respect to parameters such as pH, temperature, contact time
and/or improper recirculation flow rates) and delays in CIP application. Poorly applied
membrane cleaning procedures will limit the recovery of the system to design operating
parameters (flux, pressure drop and permeate quality) and incomplete removal of biomass
may accelerate the growth of bacteria in other parts of the system.
3. Genesol 703 – new cleaning approach
Cleaning agents can affect fouling materials present on a membrane surface in three ways: (i)
foulants may be removed, (ii) morphology of foulants may be changed (swelling,
compaction) and/or (iii) surface chemistry of the deposit may be altered, such that the
hydrophobicity or charge is modified [10]. Reported foulant-cleaning agent reactions are
hydrolysis, peptization, saponification, solubilisation, dispersion (suspension) and chelation.
If an inappropriate cleaning agent is chosen negative effects can appear and membrane
performance can be adversely affected. Membrane manufacturers [11] clearly state the
consequences of applying inefficient cleaning techniques: “If foulant is not successfully
removed, the membrane system performance will decline faster as it is easier for the foulant
to deposit on the membrane surface area. The time between cleanings will become shorter,
resulting in shorter membrane element life and higher operating and maintenance costs.Most
effective cleaning allows longer system operating time between cleanings and results in the
lowest operating costs”.
Genesol 703 was developed and tested as an effective cleaning compound for the removal of
clay deposits [12]. Genesol 703 is a 100% active chemical powder based on a combination of
high pH phosphate cleaners, a blend of chelants, surfactants and other active compounds. The
product is approved under NSF/ANSI 60 guidelines.This combination of products has a
detergent and surfactant effect on the colloidal foulants and in addition creates high ionic
strength at the membrane surface. Due to the relevance of biofouling problems detected in
Genesys Membrane Products laboratories (detected in 35% of membranes autopsied) and
synergistic properties of this formulated cleaner, trials against biofilm removal under
laboratory conditions were conducted.
The Genesol 703 mode of action can be described as follows: the first stage of attack occurs
at the water/surface inter-phase of the biofilm and is due to the synergistic mode of operation
of the combined speciality chemicals. This process works by reducing the surface tension of
the deposit allowing the surfactant to become more effective in overcoming the
impermeability of the EPS material; this allows the cleaning solution to penetrate into the
biofilm structure. The foulant layer then becomes more porous increasing the permeability to
water and consequently increasing the surface area of the deposit allowing more active
chemical to penetrate and disrupt the “body” of the deposit. Genesol 703 provides a
secondary physical action which increases cleaning efficiency at the membrane surface
allowing a “double edged” approach to deposit removal. This action removes blockages from
the membrane pores caused by the biofilm layer.
In normal operation of an RO system the pressure provided by the high pressure pump (HPP)
overcomes the osmotic pressure of the feed water. During cleaning, the Genesol 703 solution
is introduced to the system at a cleaning pressure below 4 bar. The feed water salinity will
increase. It is possible that at the membrane surface the local osmotic pressure may become
higher than the Net Driving Pressure (NDP) of the feed water. If this were the case then
potentially there may be some localised forward osmosis taking place. Any movement of
permeate water through the membrane to the feed water may assist lifting of the biofilm
around the membrane pores. This in turn would allow greater access to the surfactant
cleaning chemicals to remove deposits. The removal of deposits away from the membrane
into the concentrate stream is likely to help minimise membrane abrasion. This phenomenon
may go some way to explain the effectiveness of the cleaning formulation. Further work is
required to try and observe what is actually happening at the membrane surface during
cleaning.
In addition to the effectiveness of Genesol 703 in removing biofilm deposits its application
also serves as a means of “shock treatment” of a reverse osmosis system to reduce the
biofouling potential through lysis of microorganisms; in turn this helps to prevent further
system contamination. Cell lysis occurs due to the semi permeable nature of the membrane
surrounding the microorganism; the cleaning solution creates the movement of water from
the cell cytoplasm resulting in the eventual removal of the membrane from the cell wall. In
addition to removing the biofilm layer from the membrane surface this effect helps to destroy
remaining active cells preventing swift repopulation of the system.
Laboratory tests (as indicated below) proved this product to be much more effective at
removing biofouling deposits than conventional acid and alkaline cleaning products.
5. Testing Genesol 703 efficiency – Experimental set up and procedures
In order to establish the efficiency of Genesol 703 in removing biofouling from a membrane
surface, several cleaning tests were carried out in the Genesys Membrane Products S.L.
laboratories using membrane coupons from three actual RO membrane elements in which
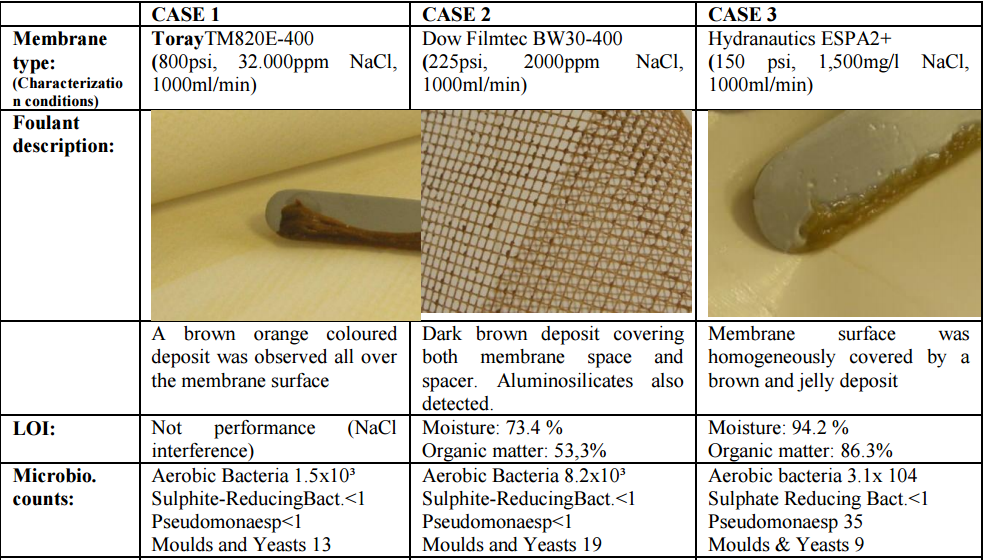
biofouling was identified. In order to verify that the deposits on these membrane elements
were mainly composed of biofilm, several observations (deposit morphology, moisture and
organic matter content) and techniques were taken into consideration:
a) Microbiological counts on membrane surface
b) Membrane surface/deposit inspection by Scanning-electron microscopy – Energy
dispersive X-ray analysis (SEM-EDAX) is used to study the membrane surface and to
verify the elemental composition of its foulant and deposits detected. Elemental
determination with the SEM-EDXA system is based on analysis of X-rays produced
via electron beam excitation of a sample area.
c) Foulant/membrane surface analysis by Attenuated Total Reflectance Infrared
Spectrometry (ATR/IR). This technique can provide valuable information related to
chemical structures and characterize the fouling layer from membrane surfaces.
In the mid-infrared, absorption of radiation is related to fundamental vibrations of the
chemical bonds. Internal reflection spectrometry provides information related to the
presence or absence of specific functional groups. IR spectra were carried out for the
foulant taken from the membrane surface in all case studies. In all of the 3 cases the
identified compounds are protein derivatives that are commonly related to the
presence of microorganisms / biofilm (bands at 1639 and 1561 cm-1
).
Data for membranes selected for this study are summarized in Table 1.
Cleaning experiments were performed with a laboratory scale cross-flow test rig unit.
Rectangular flat sheet membrane coupons from RO elements were housed in a stainless steel
cell, with an effective membrane area of 231cm2
. Feed water was circulated under the
characterisation conditions (pressure and salinity) established by the membrane element
manufacturer in order to establish a baseline for each membrane sample. Data achieved is
normalized to 25ºC conditions. Different cleaning solutions were later re-circulated at 40 psi.
The cleaning chemical used on each membrane and the test conditions are described below.
After re-circulating the cleaning solution the membrane is rinsed with deionised water and
characterised with the same conditions as used in step one. The cleaning efficiency of the
product is then evaluated in terms of flux and rejection percentual variations. Additional
analysis and visual inspection can be carried out to provide further evaluation.
6. Testing Genesol 703 efficiency – Results and discussion
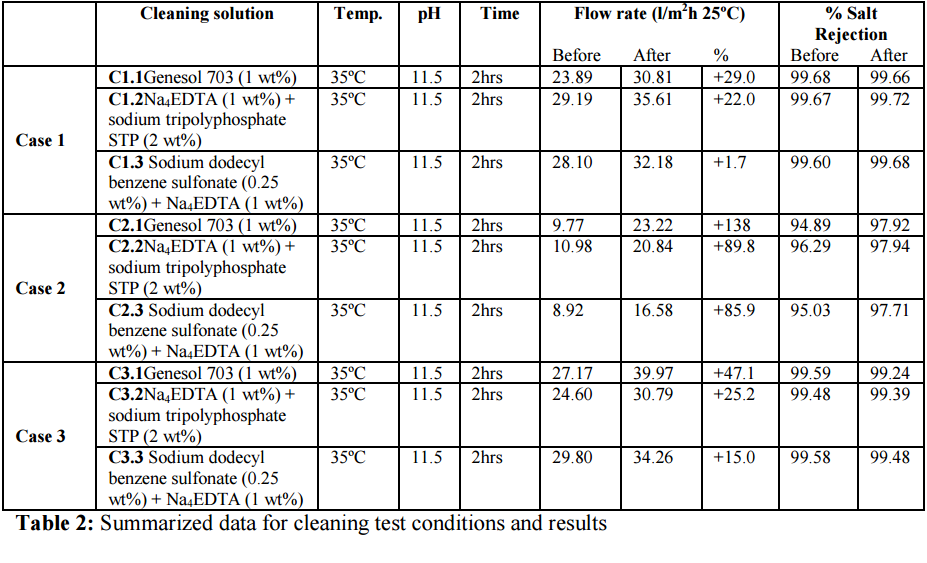
In order to check Genesol 703 efficiency, several cleaning programs were designed according
to the membrane manufactures guidelines for removing this type of foulant. Established
limits of pH and temperature have also been applied. In order to achieve comparative results,
contact time in each trial has been set up in 2 hours. The results obtained in the different
cleaning tests and the conditions applied (temperature, pH and contact time) are summarized
in Table 3. Figure 3 depicts a graphical summary from different case studies showing the
percentage flux change of each membrane coupon section after the cleaning process.
Individual chemical cleaning solution relate to those shown in table 3.
The data obtained in this experimental work demonstrates that Genesol 703 is more efficient
in removing this kind of biological foulant than the other chemical blends in term of flux
improvements. With regards to the evaluation of salt rejection data the results are
inconclusive – as in most cases a decrease in salt rejection was observed after chemical 611 925 1044 1235 13181408 1379 1453 1544 1655 2852 2924 3399
GA071136
PROTEINS FROM URINARY SEDIMENTS
1.3 Absorbance
4000 3500 3000 2500 2000 1500 1000 500
Wav enumbers (cm-1)
cleaning. It is important to point that in this experiment fouled membrane samples come from
real plants and they have been operating under fouling conditions a variable period of time in
each case. Although biofilm has been documented as more significant fouling in these
samples, it is widely assumed that biological fouling is seldom found alone (“composite
fouling”) and its properties for enhancing particulate fouling.A reasonable explanation to
these results would be membrane abrasion which is frequently documented in membranes
fouled by colloids [12] and operation under high differential pressure conditions.
read more at
http://www.genesysro.com/uploads/docs/GenesysInternational-RemovingBiofilmfromMembranes.pdf